- Bulgaria-Italy-Spain supercapacitors


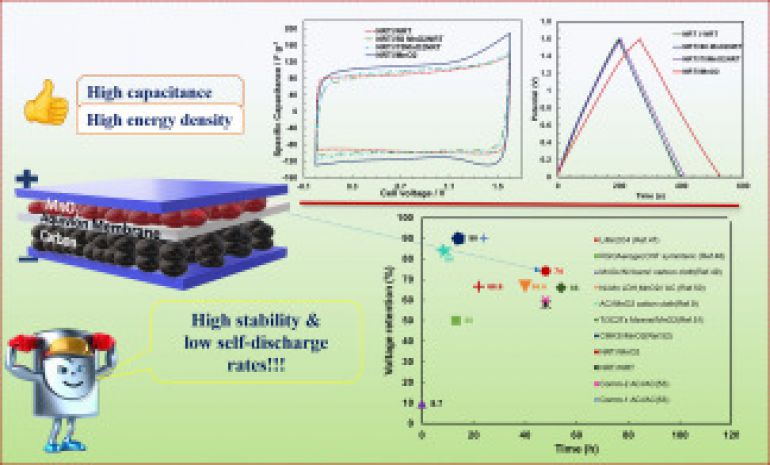
In this research work, we reported the synthesis of MnO2 by a simple co-precipitation technique and its use as electrode material for supercapacitors. Thus, hybrid supercapacitors with asymmetric configuration were constructed using a commercial activated carbon as the negative electrode, MnO2 and composites in the positive electrode, and Na+-form Aquivion electrolyte membrane as the separator and polymer electrolyte. The synergistic influence of MnO2 and carbon in the electrodes resulted in perfectly reversible charge storage processes at the positive electrode. As a result, the fabricated solid-state supercapacitors exhibited well rectangular voltammograms and a high specific capacitance of 130 F g ̶ 1 at 0.2 A g ̶ 1 , as well as an energy density of 11.5 Wh kg ̶ 1 at a power density of 161 W kg ̶ 1. Moreover, this type of hybrid supercapacitor was able to achieve a long lifetime (i.e., 150 h at 1.6 V) for up to 10,000 cycles through a combination of galvanostatic charging/discharging, and floating conditions without compromising the capacitance stability. In addition, the NRT//MnO2 supercapacitor exhibited excellent voltage retention (self-discharge) of more than ~74% at 48 h after charging in a high-voltage window of 1.6 V. These results suggest that the presented asymmetric solid-state configuration may be suitable for developing more efficient and sustainable energy storage devices.